
How to Evaluate Pharmaceutical Doors Manufacturer for Reliability
- By:Lisa
- 2026-02-04
- 29
Imagine a pharmaceutical facility. One broken seal on pharmaceutical doors can let in contaminants. This can halt production and put patients at risk. Delays can worsen, leading to expensive recalls. These issues are prevalent, with over 30% of contamination cases stemming from door seal failures or malfunctioning doors.
- Doors that are not reliable can impede critical work.
- Choosing an unreliable supplier can result in project delays.
- Managing risks starts with selecting the right partner.
E-ZONG has many years of experience and sets a high standard in the field of pharmaceutical doors.
What Defines Reliable Pharmaceutical Doors
Risk Mitigation and Compliance
Reliable pharmaceutical doors help lower risks in clean rooms. Every door should follow strict rules. These rules keep your facility safe from contamination and fines.
Here is a summary of the main rules for pharmaceutical doors:
| Compliance Benchmark | Description |
|---|---|
| Material Restrictions | GMP rules do not allow wooden doors. Good materials are fiberglass and stainless steel. |
| Cleanability | Doors should be easy to clean to keep things sanitary. |
| Performance | Strong doors protect products from harmful things. |
| Compliance with GMP | Doors must follow Current Good Manufacturing Practices to keep products safe. |
You should ask for papers that show these rules are followed. This means certificates for GMP, ISO, and other important standards. Good manufacturers give clear records for every door they make.
Preventing Contamination and Delays
Pharmaceutical doors do more than just close a room. They block dust, germs, and other bad things. If a door breaks, contamination can spread fast. This can stop production, waste supplies, and cause recalls.
To stop these problems, choose doors with:
- Airtight seals that stop air leaks.
- Surfaces that do not stain and are easy to clean.
- Strong frames that do not bend or break.
Checklist:
- Does the door have a paper showing it is airtight?
- Are cleaning steps and care plans given?
- Can the supplier show proof of good work in other places?
If you pick doors with these features, you lower the chance of contamination and delays. Reliable pharmaceutical doors help you reach your quality goals and finish work on time.
Why Reliability Is Critical in Pharmaceutical Facilities
Contamination Control
Contamination is a big problem in pharmaceutical places. A small crack or broken seal in a door can let in dust or germs. This can spoil medicine and make it unsafe for patients. Clean rooms must always stay controlled. Pharmaceutical doors are very important for this job. They need to follow strict rules to keep out bad things.
Good doors help places follow rules like Current Good Manufacturing Practices (cGMP). These rules say doors must seal well and be easy to clean. Picking reliable doors protects your products and your company’s name. It also lowers the chance of expensive recalls or shutdowns.
Project Delivery Risks
Bad doors can cause more problems than just contamination. They can slow down your whole project. If a door breaks when being put in or used, work may have to stop. This can make you miss deadlines and spend more money. Delays can mess up other things, like HVAC systems or cleanroom checks.
A good supplier will bring doors on time and give clear plans. They will help if something goes wrong. When you build a new place or fix an old one, ask these questions:
- Has the supplier done this kind of work before?
- Can they show proof or stories from other jobs?
- Do they give clear schedules and updates?
Picking reliable pharmaceutical doors helps you avoid problems and finish your project on time. It keeps your facility safe, clean, and ready to work.
Regulatory Compliance for Pharmaceutical Doors
Certification and Standards
Regulatory compliance is very important in pharmaceutical places. Every door must follow strict rules from groups like the FDA, ISO, and GMP. These rules help keep your facility safe from contamination. They also help you pass checks and audits. The right doors help control the environment and lower risks.
Here is a table with the main needs for pharmaceutical doors:
| Requirement Type | Description |
|---|---|
| Surface Material | Smooth surfaces stop bacteria from growing. |
| Corrosion Resistance | Materials must work well in wet or humid places. |
| Design Features | Seamless designs keep out dirt and germs. |
| Mechanisms | Self-closing or tight seals keep air in and out. |
| Cleanability | Doors should be easy to clean and handle strong washing. |
Look for doors with airtight seals and antimicrobial finishes. Electrostatic-dissipative surfaces are also good. These features help keep the area sterile. They also help you follow cGMP, FDA, and ISO rules. Following ISO rules helps you get ready for audits and meet world standards.
Documentation and Audit Support
Good paperwork is needed for compliance. Always ask your door supplier for certificates and records. These papers should show the doors meet GMP, FDA, and ISO rules. Ask for:
- Certificates for each door model
- Test results for airtightness and cleaning
- Records that track materials and how doors are made
- Instructions for cleaning and care
A good manufacturer will give you clear and full paperwork. This makes passing checks easier and keeps your facility safe and following the rules.
Material Quality and Cleanability
Durable Materials
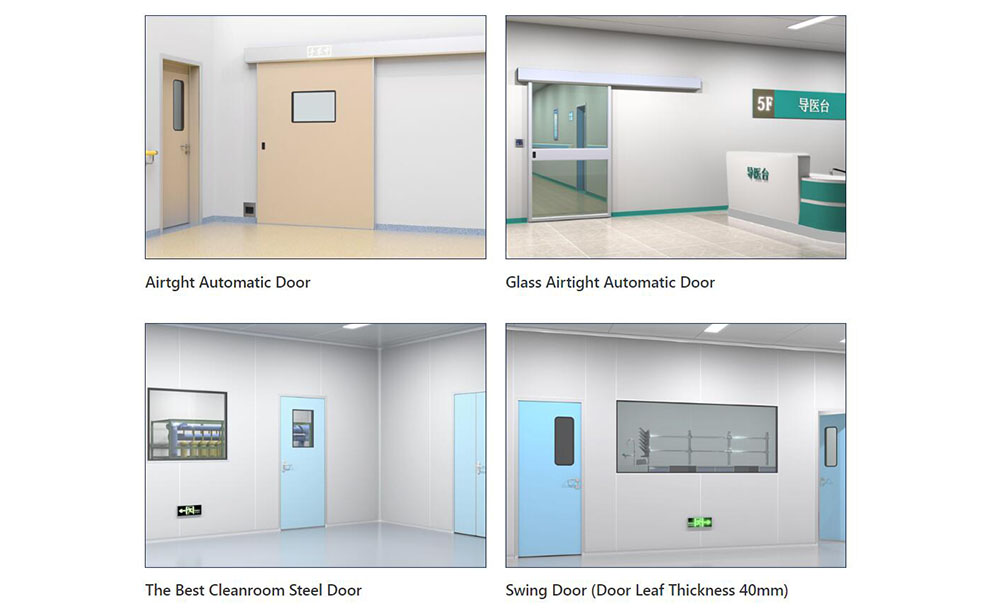
Picking the right material for pharmaceutical doors is very important. It helps keep the doors strong and stops contamination. The materials must handle lots of cleaning and chemicals. They also need to be tough against bumps and hits. Do not use wood because it does not follow GMP rules. Wood also needs a lot of care. Use doors made from fiberglass, stainless steel, or glass-reinforced plastic (GRP). These materials fight off water, chemicals, and damage. They work well in cleanrooms.
Key properties for pharmaceutical door materials:
| Property | Critical Requirement | Sterilization Method Driver |
|---|---|---|
| High Temperature Resistance | Handles heat from 121°C to 135°C | Autoclaving (steam/heat) |
| Low Compression Set | Bounces back after being used | All methods |
| Chemical Resistance | Stays strong with cleaning agents | Facility protocols |
| Minimal Extractables | Keeps products safe | Product safety |
| Hydrolytic Stability | Stays strong in steam | Autoclaving |
- Fiberglass and stainless steel doors last a long time and follow the rules.
- GRP doors do not get ruined by chemicals or water.
- Laminate and wood are old choices and do not work for cleanrooms now.
Tip: Make sure the door material matches how you clean and sterilize things in your facility.
Easy-to-Clean Design
It is very important to keep pharmaceutical doors clean. The design should not have many seams or places for dirt to hide. Smooth surfaces and tight seals help stop germs and dust. They also make cleaning much easier.
Features that support easy cleaning:
| Feature | Description |
|---|---|
| Materials | Surfaces that do not soak up water and fight rust and chemicals |
| Seals | Seals all around to block air and dirt |
| Design | Simple shapes with few joints or edges |
| Integration | Fits into cleanroom walls to help control air |
- Good doors are easy to fix and take care of.
- Tight seals keep the room clean and stop air leaks.
- Materials that do not rust help the doors last longer and save money.
Sealing Technology and Performance
Airtight Seal Features
Airtight seals are very important for pharmaceutical doors. These seals help keep cleanrooms free from contamination.
- Double-gasket seals close tightly on every side. They block dust and germs from getting in.
- Fast-closing doors make less air move around. This helps stop bad things from spreading.
- Aluminum and stainless steel are good materials. They do not have cracks where dirt can hide.
How fast the door opens and closes is also important. Doors that slide quickly or use sensors stay open for less time. This lowers the chance of outside dirt coming in.
Tip: When you look at doors, ask what kind of seal they use. Find out how it helps keep the room clean.
Comparison of Seal Types:
| Seal Type | Key Features | Suitability for Cleanrooms |
|---|---|---|
| Mechanical | Uses physical contact for sealing | Good for basic environments |
| Inflatable | Expands to fill gaps, adapts to shape | Best for high-pressure areas |
| Magnetic | Uses magnets for closure | Suitable for low-traffic zones |
Failure Modes and Suitability
Door seals can break in different ways. Losing sterility is a big problem. Sometimes pressure or vacuum seals stop working. This happens more in busy places. If seals need fixing a lot, work can get interrupted. Manufacturers use inflatable seals to fix these problems. Inflatable seals fit many shapes and keep air out, even with high pressure. They are also easier to clean and take care of. This helps keep the cleanroom safe.
Checklist:
- Does the seal keep things sterile all the time?
- Is the seal simple to clean and fix?
- Can the seal handle pressure changes without leaking?
Customization and Engineering Support
Tailored Solutions
Every pharmaceutical facility needs something special. Regular doors might not work for your cleanroom. Customization lets you pick doors that fit your needs. You can choose the material, size, and finish you want. This makes things safer and lowers risks.
Here is a table that shows common ways to customize pharmaceutical doors:
| Feature | Details |
|---|---|
| Construction | Double skin doors, with or without view panels |
| Shutter Thickness | 42 mm, 44 mm, 46 mm |
| Paint / Finish | Powder-coated or stainless steel |
| Panel Thickness | 50 mm, 80 mm, 100 mm, 150 mm |
| Width Variants | 750 mm to 2200 mm |
| Accessories | Hinges, handles, push plates, closures, locking systems |
| Insulation Materials | PUF, PIR, ROCKWOOL for thermal and acoustic insulation |
You can also pick:
- Special materials and sizes for your doors
- Manual or automatic ways to open doors
- Single, double, sliding, or swing-out door types
- Touchless sensors for easy opening
- Doors that fit with your cleanroom walls and systems
Tip: Ask your supplier what options they have. Get drawings or samples to make sure the doors will fit.
Integration and Validation
Doors need to work well with other cleanroom parts. They must fit with walls, floors, and air units. This stops leaks and keeps the room safe. Validation checks if the doors meet all rules after they are put in.
Important steps for integration and validation:
- Look at technical drawings with your engineers
- Make sure doors work with HVAC and access systems
- Ask for reports and test results from your supplier
- Plan inspections before and after doors are installed
Good integration and validation help stop contamination and delays. Always save records of these steps for audits and future fixes.
Operational Efficiency and Access Control
Fast Opening and Closing
Pharmaceutical facilities need doors that open and close fast. This helps keep cleanrooms safe. When doors move quickly, they do not stay open long. This lowers the chance of contamination. Workers can go from room to room without waiting. Fast doors help keep air pressure and temperature steady.
Quick access makes work easier. Staff can finish jobs faster. Production lines do not slow down. Fewer outside particles get into the cleanroom. Fast door systems help keep things clean. They also help meet rules for stopping contamination.
Tip: Ask your supplier how fast the doors work. Get proof about opening and closing speeds. This helps you compare and pick the best doors for your place.
Interlocks and Security
Access control is very important in pharmaceutical places. Interlocks and security tools protect important areas. Interlocks stop two doors from opening at once. This keeps cleanrooms closed and stops cross-contamination. Only staff with permission can enter special zones.
Security systems use maglocks and control panels. These tools help track who comes in and out. Cabinets and drawers with small maglocks keep medicine safe. Access control lets only certain people open doors, based on their job or the drug type. These features help follow industry rules.
| Feature | Contribution to Compliance and Safety |
|---|---|
| Interlock Solutions | Regulate access to critical areas, ensuring only authorized personnel can enter. |
| Compact Maglocks (Mini-Mites) | Protect cabinets and drawers where medications are stored, enhancing security. |
| Access Control Mechanisms | Limit access based on application, type, and quantity of drugs, ensuring compliance with regulations. |
Good access control means you need fewer workers. Managers can watch all doors from one spot. This saves money and makes things safer.
| Improvement Type | Description |
|---|---|
| Smoother process management | Enhances workflow efficiency within the facility. |
| Easy movement around non-sensitive areas | Facilitates access without hindrance. |
| Reduced staffing requirements | Lowers operational costs by minimizing personnel. |
| Centralized control of all gates | Streamlines management from a single location. |
Project Management and Delivery Reliability
Planning and Scheduling
Good project management helps you avoid delays. When picking a manufacturer for pharmaceutical doors, check their history. Reliable partners give clear timelines and follow them. They share simple schedules for design, making, and delivery. This helps you plan and stops last-minute problems.
Ask these questions when you check suppliers:
- Do they deliver on time most of the time?
- Can they show proof from other jobs like yours?
- What do they do if something is late?
A good manufacturer gives updates often. They tell you about each step. This keeps your team ready and lowers the chance of going over budget.
Supply Chain Transparency
Knowing where your doors are helps them arrive on time. You should know each step in the process. Manufacturers should use tracking and give updates right away. This helps you find problems early and fix them fast.
Serialization means giving each product a special number. This helps find fake items and makes recalls easier. Using technology helps track things better and keeps the supply chain safe.
Pick suppliers who use digital tracking and show where materials come from. Ask for papers that show your order’s journey from start to finish. This kind of openness builds trust and helps you control risks.
A reliable supplier lets you check progress easily. They share info and answer questions fast. This helps your project go smoothly and keeps your money safe.
Customer Service and Technical Support
Responsive Support
You need a manufacturer who answers fast when there are problems. Quick help can stop small problems from turning into big ones. If you wait too long for answers, your work may stop. This can cause contamination or make you miss important deadlines. Good support teams answer your questions and give advice. They help you fix problems in person or from far away.
Key criteria for evaluating support:
- How fast do they answer technical questions?
- When is support available (all day or just work hours)?
- Can you talk to technical experts?
- Are there language choices for teams in other countries?
Tip: Ask how long it takes them to reply and for stories about past help. Get a list of support contacts before you sign any contract.
A good support team helps your facility work well. They give clear steps and help you solve problems. You should always feel sure that help is there when you need it.
CAPA and After-Sales Service
Corrective and Preventive Action (CAPA) is very important in this field. CAPA makes sure problems are fixed and do not come back. After-sales service means help with repairs, spare parts, and upgrades. These services keep your doors working and lower future risks.
Questions to ask manufacturers:
- How do you handle CAPA requests?
- What steps do you take to fix problems?
- Do you offer regular check-ups or emergency repairs?
- How fast can you send spare parts?
| Service Type | What to Look For |
|---|---|
| CAPA Process | Clear steps for issue resolution |
| Maintenance Support | Regular checks and fast repairs |
| Spare Parts Supply | Quick delivery and easy ordering |
| Upgrade Options | Support for future compliance needs |
Note: Ask for papers about CAPA and after-sales promises. Look at sample reports to see what kind of help you will get.
Good customer service and technical support help you avoid problems. They help you follow the rules and keep your cleanroom doors working well.
To prevent contamination and meet strict regulatory standards, partnering with a reliable pharmaceutical door manufacturer is essential. E-ZONG delivers the reliability and precision needed to keep your production on track. Explore our range of solutions to find the perfect fit for your facility.
FAQ
What certifications should pharmaceutical doors have?
Pharmaceutical doors need GMP, ISO, and FDA certifications. These show the doors are safe and high quality. Always ask your supplier for current certificates.
How do I verify a door’s airtightness?
Ask the manufacturer for new test reports. These reports show if air leaks out or stays in. Check the results to see if the door fits your cleanroom.
What materials are best for cleanroom doors?
Stainless steel, fiberglass, and GRP are the best choices. These materials do not get damaged by chemicals or water. They are strong and easy to clean.
Why is documentation important during audits?
Documentation shows you follow the rules. Auditors look at certificates and test results. Good records help you pass checks and avoid fines.
How can I compare different door suppliers?
Make a table to compare each supplier. Give scores for rules, sealing, delivery, and help. This makes it easier to pick the best one.
-
Cleanroom Glass Windows Are The Key to Maintaining a Clean Environment
-
Top Aluminium Profile Manufacturers in China: Leading the Global Market
-
The Evolution of Air Tight Sliding Doors
-
AHU Aluminium Profile: A Comprehensive Guide
-
The Importance of Choosing the Right Cleanroom Door in Vietnam
-
The Benefits of Hospital Automatic Doors: Enhancing Efficiency and Safety
-
The Best Bathroom Door Manufacturers - Unlocking Endless Possibilities!
-
Unlock the Possibilities with AJ Manufacturing Doors
-
Make a Statement with Manufactured Home Interior Doors!
-
what is aluminum profile? Aluminum Profiles for Your Home is the best option
-
How to Evaluate Pharmaceutical Doors Manufacturer for Reliability
-
Automatic Door vs Manual Door for Cleanrooms: Which Is Better
-
Steel vs. Glass Sealed Door for Modern Cleanroom Needs
-
Double Swing Door vs. Single: Which Fits Your Cleanroom Needs
-
Unit Ventilator Explained: Meeting Modern HVAC Needs
-
Supply vs. Return HVAC Grilles: Healthcare Selection Guide
-
Linear Diffuser: The Secret to Modern HVAC Comfort & Design
-
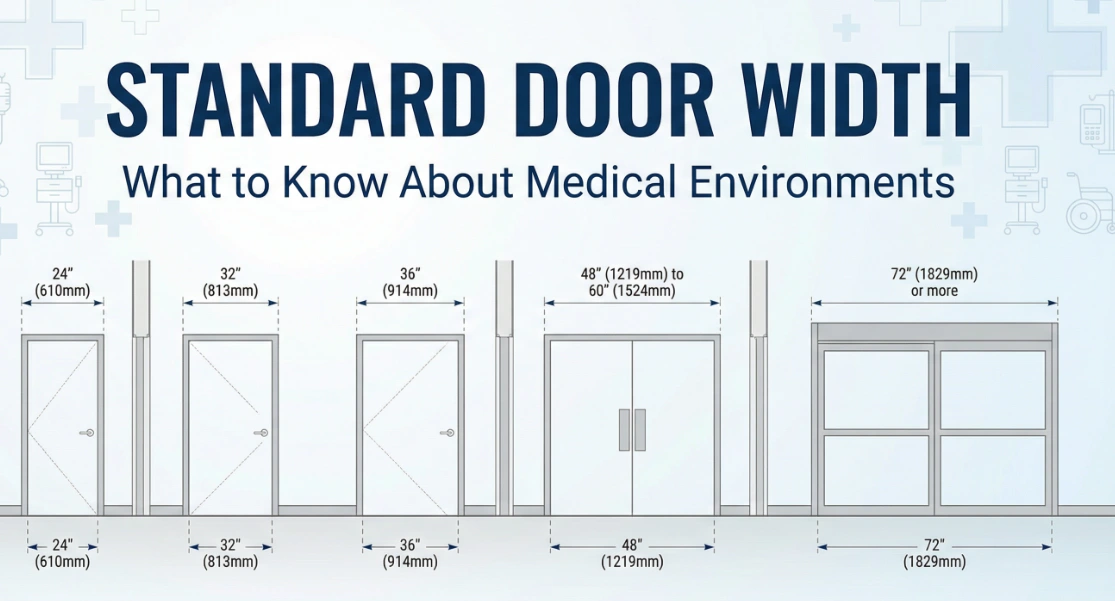
What to Know About Standard Door Width in Medical Environments
-
What Is a Return Air Vent and Why Does It Matter in HVAC
-
Top Materials for Durable Pharma Clean Room Doors

Guangzhou Yizhong Aluminum Industry Co., Ltd.
We are always providing our customers with reliable products and considerate services.
We are always providing our customers with reliable products and considerate services.





.jpg)

















Speak Your Mind