
Hospital Cleanroom Door Design Requirements
- By:Lisa
- 2025-12-24
- 29
In hospital environments, cleanroom doors serve as crucial barriers against airborne contaminants, ensuring sterile conditions in operating rooms, pharmaceutical suites, and isolation wards. Their importance is underscored by data: surgical site infections, for instance, account for a significant portion of hospital-acquired cases. To combat this, cleanroom doors must prioritize airtight seals, durable materials, and compliance with internationally recognized cleanroom standards, helping protect patients while keeping daily operations efficient and predictable. This guide offers practical insights for facility managers and architects seeking reliable solutions.
The Vital Role of Cleanroom Doors in Hospital Safety
Think of hospital cleanroom doors as active guardians, not just barriers. They defend against invisible threats—dust, microbes, airborne particles—that could turn routine care into a complication. Picture an operating theater: without proper doors, contaminated air from hallways could slip in, raising infection risks. Data from the CDC highlights that SSIs contribute to prolonged stays, adding significant treatment costs and operational pressure, often tied to lapses in environmental control.
For busy hospital teams facing tight budgets and regulatory pressures, the right doors can make all the difference. They help sustain positive pressure, where cleaner air flows outward like a protective shield, reducing energy waste and downtime.
Materials and Construction
Selecting materials for hospital cleanroom doors is like building a sturdy fence around a garden—it needs to withstand weather (or in this case, daily cleanings) while keeping unwanted visitors out. The goal is durability, ease of cleaning, and low particle generation, all while fitting the specific needs of ISO-classified spaces.
Common Materials: Pros, Cons, and Applications
Materials must comply with ISO 14644, the global standard used to define cleanliness levels in hospital cleanroom environments. In practice, most hospital cleanroom doors are designed for ISO Class 5–8 areas, where maintaining stable, low-contamination conditions is critical.
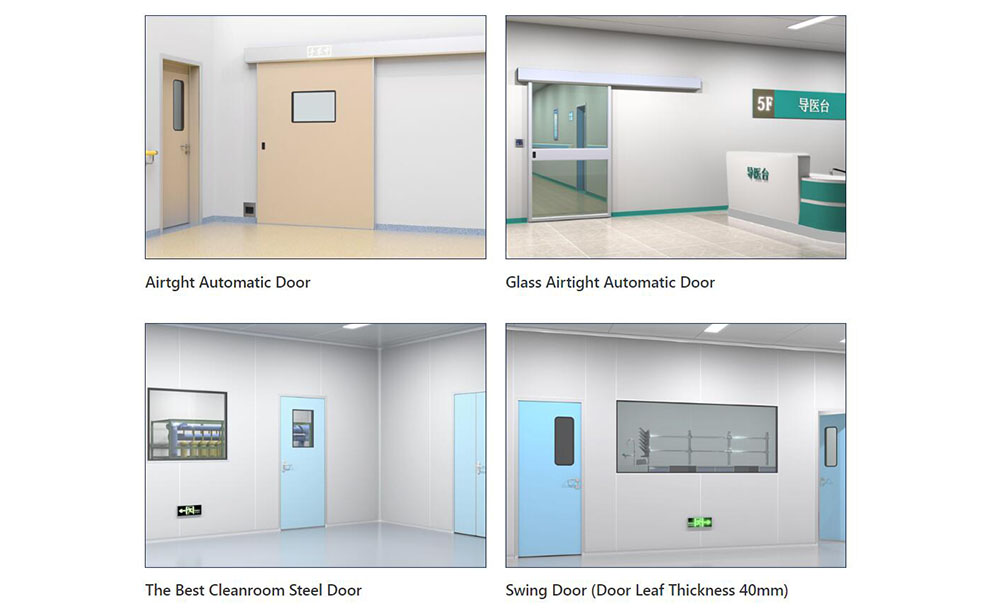
- Stainless Steel (Typically 304 or 316L Grades): This material is a favorite for high-traffic areas because it fights off rust and scratches from harsh disinfectants. It's like the tough outer shell of a turtle—strong and long-lasting, making it a reliable long-term solution for critical hospital cleanroom areas. In pharmaceutical compounding rooms, it meets strict pharmaceutical and hospital hygiene requirements by minimizing particle shedding and supporting consistent and repeatable hospital cleaning routines. However, it's heavier and pricier, so pair it with lightweight frames for balance.
- Anodized Aluminum: Lighter and more affordable, this is ideal for general hospital cleanrooms where weight matters during installation. The anodizing process creates a protective layer, much like a raincoat for metal, resisting oxidation in humid settings. It can reduce door weight by 30% compared to steel, making retrofits easier, but it might need extra coatings for extreme chemical exposure.
- Laminated Glass: When visibility is key, like in observation doors for intensive care, this option provides impact resistance without shattering, per ANSI Z97.1. It's like a clear window in a fortress—safe and functional, allowing staff to check in without opening the door and risking air exchange. Double-glazed versions add insulation, helping maintain stable temperatures.
- Composite Materials (Such as FRP or HPL): These hybrids offer flexibility and thermal properties. Good thermal insulation, helping maintain stable indoor conditions. Think of them as layered sandwiches—strong cores wrapped in protective skins—that resist warping and provide built-in antimicrobial features. They're great for modular setups in hospitals, often offering noticeable cost savings compared to all-metal door solutions.
Enhancing Cleanability Through Smart Design
Cleanability isn't just about materials; it's about thoughtful features that make maintenance straightforward. Smooth, finely finished surfaces — comparable to a non-stick pan — allow dust and microbes to be wiped away easily, even during frequent hospital-grade cleaning. Radius corners curve away sharp edges, helping reduce areas where dirt and microbes tend to accumulate over time. Seamless construction eliminates crevices where germs hide, aligning with widely accepted hospital and pharmaceutical hygiene practices. Chemical resistance ensures doors can withstand frequent, long-term hospital-grade cleaning, like a well-worn pair of boots that still look new.
In practice, these designs address everyday challenges: a hospital lab door with seamless stainless steel can be sanitized in minutes, cutting labor costs and infection risks.
Size, Clearance, and Accessibility: Practical Considerations
Standard sizes start at 900mm x 2100mm for personnel, scaling up for equipment access to avoid bottlenecks. Clearances are tightly controlled to minimize gaps and ensure effective sealing without affecting smooth operation. Accessibility complies with ADA, featuring lever handles and low thresholds for wheelchairs. Custom sizing, enabled by precise manufacturing, fits unique layouts and can shorten installation by 20-30%.
To help compare, here's a detailed table based on industry benchmarks:
| Material Type | Corrosion Resistance | Particle Shedding Risk | Installation Ease | Typical Hospital Use Case | Cost & Efficiency Considerations |
|---|---|---|---|---|---|
| Stainless Steel | Excellent | Low | Moderate | Surgical suites | Long-term durability offsets initial high cost |
| Anodized Aluminum | Good | Low | High | General wards | 30% lighter, faster setup |
| Laminated Glass | Moderate | Very Low | Medium | Isolation monitoring | Reduces unnecessary door openings, helping lower contamination risk |
| Composites | Excellent | Low | High | Pharma storage | 15-25% upfront savings, energy efficient |
These elements not only meet technical specs but solve pain points like budget constraints and compliance deadlines, drawing from industry comparisons for real-world applicability.
Airtight Sealing and Pressure Control
Airtight sealing is the unsung hero of hospital cleanrooms, ensuring pressure differences that keep contaminants out—like a dam holding back a river. Hospitals typically maintain gentle positive air pressure, so clean air naturally flows outward rather than allowing corridor air to enter. In many hospital cleanroom areas, this pressure difference is carefully controlled and continuously monitored.
Effective Sealing Mechanisms and Hardware
Seals come in varieties to suit different needs, all tested under standards like EN 12207 for permeability.
- Magnetic Seals: These use continuous strips for a snug fit, perfect for ISO Class 7/8 rooms with very low air leakage, even under frequent daily use. They're reliable for frequent use, like in busy wards.
- Inflatable Seals: Expanding with air or gas, they create dynamic barriers for high-differential environments, such as negative pressure isolation for infectious patients.
- Double Sealing Systems: Redundant layers provide failover, essential in critical pharma applications where a single failure could contaminate batches.
- Automatic Door Operators: Sensor-activated for touchless entry—imagine doors opening with a wave—reducing manual contact and contamination by up to 40%, keeping hands sterile.
Hardware integration, like interlocking systems, prevents simultaneous openings, maintaining pressure integrity.
Pressure Differential Management in Action
A small but stable pressure difference is maintained to ensure clean air flows outward, monitored with real-time sensors and alarms to catch deviations quickly. Airlock setups act as buffers, with doors that lock until pressure stabilizes. For example, in pharmaceutical compounding, this prevents even brief lapses that could spoil products, as per EU GMP Annex 1's risk-based approach.
Insulation and Preventing Air Leaks
Well-designed thermal insulation helps minimize heat transfer and energy loss, supporting energy efficiency in temperature-controlled zones. Air leakage tests per EN 12426 confirm water tightness, while in-house validations ensure doors meet benchmarks.
Visualize this with a simple chart:
| Pressure Level | ISO Class | Minimum Differential (Pa) | Common Hospital Application |
|---|---|---|---|
| Positive | 5-6 | 15+ | Operating rooms |
| Positive | 7-8 | 10-15 | Pharma suites |
| Negative | 7-8 | -10 to -15 | Isolation wards |
These strategies offer ROI by lowering SSI rates—facilities with robust sealing report 15% energy savings and fewer infections, addressing operational pains like tight schedules.
Compliance with Hospital Cleanroom Standards
Navigating standards is like following a recipe for a perfect cake—skip a step, and it falls flat. Compliance prevents penalties, which affect 10-20% of facilities annually, ensuring doors contribute to clean air and safety.
ISO 14644 and cGMP: The Core Frameworks
ISO 14644 classifies cleanliness by particle counts, with hospitals often in Classes 5-8 (with lower ISO numbers indicating stricter cleanliness control.). It's like grading air quality on a scale—lower numbers mean cleaner spaces. cGMP guidelines under FDA 21 CFR Part 211 require doors that facilitate cleaning and pressure control, emphasizing seamless designs to avoid mix-ups.
For pharma areas, this means validation of seals and materials to prevent biological contamination carried on airborne particles.
Local, National, and Regional Regulations
Building codes mandate fire ratings and structural integrity; Joint Commission focuses on infection control with pressure monitoring. CDC guidelines recommend negative pressure for airborne isolation. Regional variations, such as EU GMP's emphasis on risk assessment, call for flexible designs. Technical support helps tailor solutions to these.
Documentation and Certification Essentials
Mandatory items include test reports for leaks and differentials, material traceability certificates, installation guides, and maintenance logs for audits. Proper records ensure ongoing compliance, like a health checkup for your facility.
A quick list of key docs:
- Air leakage and pressure test reports
- Material compatibility certificates
- Step-by-step installation manuals
- Annual maintenance verification logs
These standards, backed by sources like FDA guidance, mitigate risks and streamline approvals.
Contamination and Sterility Control Features
Contamination control is the door's frontline defense, integrating features that trap or kill germs before they cause harm. In hospitals, where surfaces can retain pathogens post-cleaning, these elements are non-negotiable.
Particle and Microbial Control Tactics
HEPA integration allows seamless HVAC compatibility, meeting industry-recognized hospital cleanroom air filtration performance standards. Smooth, low-shedding surface finishes help reduce particle release; optional antimicrobial coatings (silver or copper-based) inhibit 99% growth, like a natural repellent.
Particle testing verifies doors don't add contaminants, aligning with ISO requirements.
Integrating with Cleanroom Protocols
Doors near gowning areas minimize traffic; pass-through designs for material transfer keep flows one-way. Personnel patterns optimize to avoid cross-contamination, while emergency breakaways ensure safety without breaches.
Optional Accessories for Boosted Protection
- Viewing Windows: Double-glazed with seals for safe observation.
- Interlocking Systems: Lock adjacent doors to maintain pressure.
- UV-C Lighting: Zaps germs on surfaces.
- Touchless Controls: Infrared or foot pedals for hands-free use.
These features, inspired by cleanroom best practices, lower risks in high-stakes settings.

Installation and Maintenance in Hospital Cleanrooms
Installation and maintenance keep doors performing like well-oiled machines, minimizing disruptions in fast-paced hospitals.
Installation and Retrofitting Steps
Start with site assessments to evaluate fit. Retrofits adapt to existing frames in 2-4 weeks, scheduled off-hours to avoid operational halts. Timeline: Assess (1 day), install (1-2 weeks), test (1 day).
Routine Maintenance and Cleaning
Daily: Wipe with approved disinfectants.
Monthly: Inspect seals for wear.
Quarterly: Lubricate hardware.
Annual: Verify pressure performance and overall cleanliness levels.
Accessories and Support
Replacement kits for seals, hinges, and glass; 24/7 technical help for repairs.
Consistent care reduces failures by 25%, extending ROI.
Conclusion
Hospital cleanroom doors are essential for contamination control, compliance, and patient safety. By focusing on materials, sealing, and maintenance, facilities can reduce risks and costs. A good first step is to assess your current setup. Consulting with experts can help identify the most effective upgrades for your specific needs.
-
 Cleanroom Glass Windows Are The Key to Maintaining a Clean Environment
Cleanroom Glass Windows Are The Key to Maintaining a Clean Environment -
 Top Aluminium Profile Manufacturers in China: Leading the Global Market
Top Aluminium Profile Manufacturers in China: Leading the Global Market -
 The Evolution of Air Tight Sliding Doors
The Evolution of Air Tight Sliding Doors -
 AHU Aluminium Profile: A Comprehensive Guide
AHU Aluminium Profile: A Comprehensive Guide -
 The Importance of Choosing the Right Cleanroom Door in Vietnam
The Importance of Choosing the Right Cleanroom Door in Vietnam -
 The Benefits of Hospital Automatic Doors: Enhancing Efficiency and Safety
The Benefits of Hospital Automatic Doors: Enhancing Efficiency and Safety -
.jpg) The Best Bathroom Door Manufacturers - Unlocking Endless Possibilities!
The Best Bathroom Door Manufacturers - Unlocking Endless Possibilities! -
 Unlock the Possibilities with AJ Manufacturing Doors
Unlock the Possibilities with AJ Manufacturing Doors -
 Make a Statement with Manufactured Home Interior Doors!
Make a Statement with Manufactured Home Interior Doors! -
 what is aluminum profile? Aluminum Profiles for Your Home is the best option
what is aluminum profile? Aluminum Profiles for Your Home is the best option
-
 Hospital Cleanroom Door Design Requirements
Hospital Cleanroom Door Design Requirements -
 Swing Doors vs Standard Doors: Which Is Right for Your Facility
Swing Doors vs Standard Doors: Which Is Right for Your Facility -
 Cleanroom Door Materials Explained: Key Types and Applications
Cleanroom Door Materials Explained: Key Types and Applications -
 How Do Different Vent Grills Impact Medical Cleanrooms
How Do Different Vent Grills Impact Medical Cleanrooms -
 Controlled Environment vs Air Clean Room Differences Explained
Controlled Environment vs Air Clean Room Differences Explained -
 How to Extend the Life of Your Exterior Steel Door
How to Extend the Life of Your Exterior Steel Door -
 What Are the Best Materials for AC Vent Covers
What Are the Best Materials for AC Vent Covers -
 Ceiling Vent Covers Labeled Fire-Rated? Here’s How to Verify
Ceiling Vent Covers Labeled Fire-Rated? Here’s How to Verify -
 Upgrading Lab Doors: When to Replace vs. When to Retrofit?
Upgrading Lab Doors: When to Replace vs. When to Retrofit? -
 Stainless Steel Door vs. Alternatives in Cleanrooms
Stainless Steel Door vs. Alternatives in Cleanrooms

Guangzhou Yizhong Aluminum Industry Co., Ltd.
We are always providing our customers with reliable products and considerate services.
We are always providing our customers with reliable products and considerate services.







Speak Your Mind