
Cleanroom Steel Doors: What Matters in Global Sourcing
- By:Lisa
- 2025-10-10
- 29
In pharmaceutical, biotech, semiconductor, and high-end medical facilities, a cleanroom steel door is far more than just an entryway—it’s a critical barrier that maintains pressure differentials, controls particulate contamination, and safeguards sterile environments.
As global supply chains mature, more projects are turning to overseas manufacturers for clean room doors. Yet low upfront cost doesn’t guarantee long-term value. A poorly designed or non-compliant cleanroom steel door can jeopardize ISO 14644 or GMP Annex 1 certification—triggering costly rework, delays, or even facility shutdowns.
This guide outlines a practical, five-part evaluation framework for sourcing high-performance doors—especially valuable for engineers and procurement professionals seeking stainless steel cleanroom doors or GMP compliant cleanroom doors.
1. Material & Construction: Stainless steel grades define long-term reliability

Choosing the right alloy
In environments where chlorine-based disinfectants (e.g., sodium hypochlorite) are used regularly, the choice of stainless steel grades is critical:
- 304 stainless steel: Suitable for general cleanroom zones
- 316 stainless steel: Contains molybdenum for superior pitting resistance—ideal for aseptic filling areas, hospital ORs, and high-corrosion zones
Always request a Mill Test Certificate (MTC) to verify material authenticity.
Surface finish: Why surface roughness Ra matters
- ISO 14644-5 recommends a surface roughness of Ra ≤ 0.8 μm
- Leading manufacturers use electropolishing (EP) to achieve Ra values of 0.2–0.4 μm
- Result: Up to 60% reduction in microbial adhesion compared to mechanically polished surfaces
For high-frequency cleaning areas, the cleanroom door Ra value has become a key acceptance criterion.
2. Performance Metrics: Air leakage rate is the gold standard for airtightness
Airtightness: The backbone of pressure control
Your cleanroom door specifications must include verified performance data:
- Air leakage rate(per EN 13241-1):
- Standard requirement: ≤ 0.5 m³/(h·m²) at 50 Pa
- High-criticality zones (e.g., sterile fill-finish): ≤ 0.2 m³/(h·m²)
- Always request third-party test reports with CMA/CNAS or ILAC accreditation
The cleanroom door leakage rate is one of the most reliable indicators of a supplier’s technical capability.
Safety performance: Fire and acoustic ratings
- Fire rated cleanroom doors: Required in hospitals and BSL-2/3 labs (typically EN 1634 EI60)
- Recommended solution: fire rated cleanroom steel doors that combine airtightness with fire integrity
- Acoustic performance: Aim for Rw ≥ 30 dB in noise-sensitive areas like PCR labs
3. Functionality & Integration: An interlock door system ensures system stability

Interlocks and automation
- An interlock door system prevents adjacent doors from opening simultaneously—critical for maintaining pressure cascades
- Essential in airlocks, gowning rooms, and material pass-through zones
Door type: Sliding doors are gaining traction
- Cleanroom sliding doors save space and offer smooth, quiet operation
- For high-traffic areas, consider automatic sliding cleanroom doors with integration to access control and HVAC systems
- Safety features: Obstacle detection, soft-close mechanism, and manual override during power failure
4. Compliance & Supplier Evaluation: Assess cleanroom door manufacturers rigorously
When evaluating cleanroom door manufacturers, verify:
Technical documentation
- Complete cleanroom door specifications (material grade, Ra value, leakage rate, fire rating)
- Valid third-party test reports (EN 13241, EN 1634, etc.)
GMP compliance understanding
- Can they address GMP Annex 1 (2022) requirements for “smooth, cleanable, non-shedding surfaces with no crevices”?
- Do they provide traceability documentation and installation validation support?
Customization and delivery capability
- Experience with pharmaceutical grade cleanroom doors (e.g., vision panels, oversized units)
- BIM models (Revit/IFC), installation guides, and local technical support
5. Final Thoughts: Smart sourcing starts with details—and ends with trust
When sourcing globally, ask the right questions:
- Do they provide real air leakage rate data?
- Are stainless steel grades and surface roughness Ra clearly documented?
- Can they deliver an interlock door system and fire rated cleanroom doors?
- Do they truly understand what GMP Annex 1 demands of a cleanroom steel door?
Only suppliers who excel in technical quality, regulatory alignment, and service reliability deserve your partnership.

-
 Cleanroom Glass Windows Are The Key to Maintaining a Clean Environment
Cleanroom Glass Windows Are The Key to Maintaining a Clean Environment -
 Top Aluminium Profile Manufacturers in China: Leading the Global Market
Top Aluminium Profile Manufacturers in China: Leading the Global Market -
 The Evolution of Air Tight Sliding Doors
The Evolution of Air Tight Sliding Doors -
 AHU Aluminium Profile: A Comprehensive Guide
AHU Aluminium Profile: A Comprehensive Guide -
 The Importance of Choosing the Right Cleanroom Door in Vietnam
The Importance of Choosing the Right Cleanroom Door in Vietnam -
 The Benefits of Hospital Automatic Doors: Enhancing Efficiency and Safety
The Benefits of Hospital Automatic Doors: Enhancing Efficiency and Safety -
.jpg) The Best Bathroom Door Manufacturers - Unlocking Endless Possibilities!
The Best Bathroom Door Manufacturers - Unlocking Endless Possibilities! -
 Unlock the Possibilities with AJ Manufacturing Doors
Unlock the Possibilities with AJ Manufacturing Doors -
 Make a Statement with Manufactured Home Interior Doors!
Make a Statement with Manufactured Home Interior Doors! -
 what is aluminum profile? Aluminum Profiles for Your Home is the best option
what is aluminum profile? Aluminum Profiles for Your Home is the best option
-
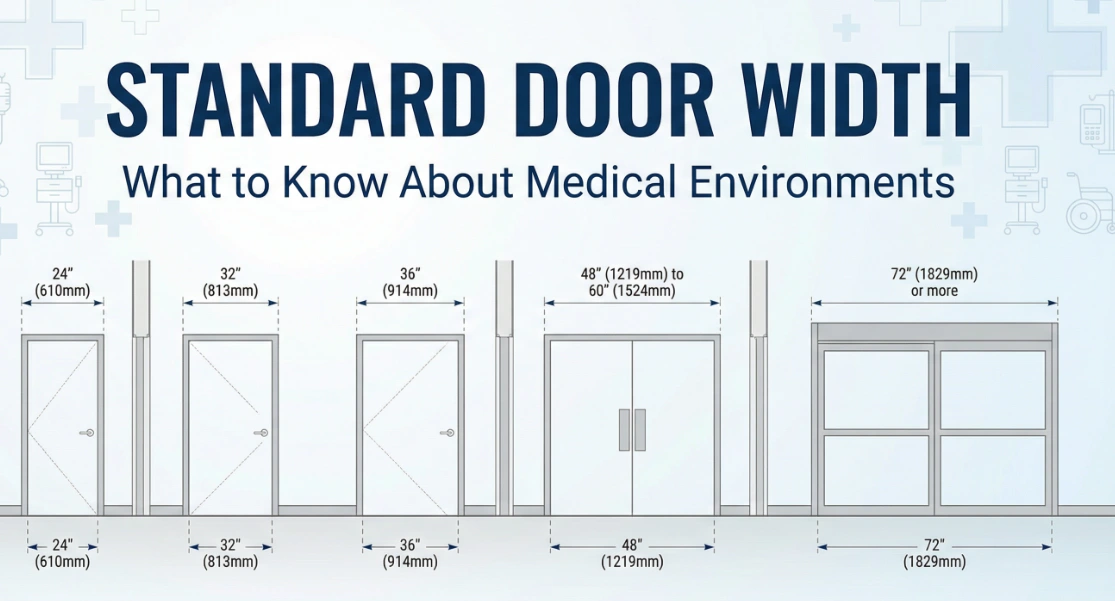 What to Know About Standard Door Width in Medical Environments
What to Know About Standard Door Width in Medical Environments -
 What Is a Return Air Vent and Why Does It Matter in HVAC
What Is a Return Air Vent and Why Does It Matter in HVAC -
 Top Materials for Durable Pharma Clean Room Doors
Top Materials for Durable Pharma Clean Room Doors -
 Hospital Cleanroom Door Design Requirements
Hospital Cleanroom Door Design Requirements -
 Swing Doors vs Standard Doors: Which Is Right for Your Facility
Swing Doors vs Standard Doors: Which Is Right for Your Facility -
 Cleanroom Door Materials Explained: Key Types and Applications
Cleanroom Door Materials Explained: Key Types and Applications -
 How Do Different Vent Grills Impact Medical Cleanrooms
How Do Different Vent Grills Impact Medical Cleanrooms -
 Controlled Environment vs Air Clean Room Differences Explained
Controlled Environment vs Air Clean Room Differences Explained -
 How to Extend the Life of Your Exterior Steel Door
How to Extend the Life of Your Exterior Steel Door -
 What Are the Best Materials for AC Vent Covers
What Are the Best Materials for AC Vent Covers

Guangzhou Yizhong Aluminum Industry Co., Ltd.
We are always providing our customers with reliable products and considerate services.
We are always providing our customers with reliable products and considerate services.







Speak Your Mind